ProteoTuner expression systems in plasmid format
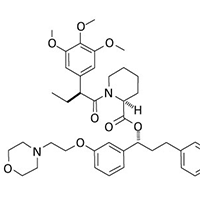
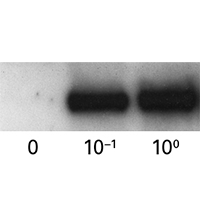
ProteoTuner plasmid expression systems allow for rapidly regulating the presence or absence of a protein of interest in your target cells. Clone your gene of interest in-frame with the destabilization domain tag (DD), either at the N or C terminus. In the absence of the Shield1 ligand in the culture medium, the DD-tagged fusion protein is immediately destabilized, but when Shield1 is added, the protein accumulates to detectable levels within 15–20 minutes.
ProteoTuner plasmid expression systems allow for rapidly regulating the presence or absence of a protein of interest in your target cells. Clone your gene of interest in-frame with the destabilization domain tag (DD), either at the N or C terminus. In the absence of the Shield1 ligand in the culture medium, the DD-tagged fusion protein is immediately destabilized, but when Shield1 is added, the protein accumulates to detectable levels within 15–20 minutes. Upon Shield1 removal, the halftime for the fusion protein's degradation can be as short as 30 minutes. Lentiviral and retroviral formats are also available.
Overview
- Rapid kinetics: protein level changes in minutes allows accurate functional analysis
- Precise tuning: precise control of protein level by controlling the dose of Shield1
- Reversible control: "protein on" to "protein off" for convincing gene-function studies
- What you get: each kit is supplied with a plasmid vector and an aliquot of Shield1.
- NOTE: Most of the proteins that we tested showed a better destabilization profile when the DD tag was fused to the N-terminus of the protein of interest (Systems N). Specific DD tag mutants for C-terminal tagging are available as well (System C); however they have a slightly reduced destabilization activity in the absence of the Shield1 ligand.
More Information
Applications
- Protein function in pathways
- Functional analysis of subunits of a protein complex
- Functional analysis of essential proteins
Additional product information
Please see the product's Certificate of Analysis for information about storage conditions, product components, and technical specifications. Please see the Kit Components List to determine kit components. Certificates of Analysis and Kit Components Lists are located under the Documents tab.
Control protein stability with Shield1
ProteoTuner systems allow quick, predictable regulation of protein presence or absence in mammalian cells by acting directly on the protein, using a small, cell-permeable synthetic compound. When Shield1 is added to the medium, any fusion protein containing a DD domain can accumulate to detectable levels within 15–20 minutes. Conversely, upon Shield1 removal, the half-time for the protein's degradation can be as short as 30 minutes. This post-translational regulation offers many benefits, from speed and convenience to precise tuning and reversible control.
Technology explained Product selection guideTakara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.