RetroNectin reagent—FAQs
Takara Bio, in collaboration with Indiana University, discovered that a recombinant human fibronectin fragment (RetroNectin reagent) dramatically enhanced the efficiency of gene transduction into hematopoietic stem cells by retrovirus vectors (Hanenberg et al. 1996). This groundbreaking discovery overturned the major premise that it is impossible to transduce genes into hematopoietic stem cells.
Click the tabs below to expand for details related to some of the most commonly asked questions about RetroNectin reagent, or visit the RetroNectin product page for ordering information.
Reference
Hanenberg, H., et al., Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat. Med. 2(8):876-882 (1996).
What is RetroNectin reagent?
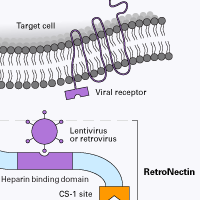
RetroNectin reagent is a recombinant human fibronectin fragment (rFN-CH-296; 574 amino acids; ~63 kD) that contains three functional domains: a cell-binding domain, a heparin-binding domain, and a CS-1 sequence. RetroNectin reagent enhances lentiviral- and retroviral-mediated gene transduction by aiding the colocalization of target cells and viral particles.
What is Fibronectin?
Fibronectin is a high molecular weight (220–250 kD) component of the extracellular matrix. Fibronectin exists as a dimer and interacts with extracellular matrix components to participate in cell migration, morphology, growth, and adhesion.
How does RetroNectin reagent colocalize viral particles and cells? Where do the virus particles bind?
RetroNectin reagent can enhance retroviral-mediated gene transfer into mammalian cells by colocalizing retrovirus particles and target cells. Colocalization is thought to be accomplished by direct binding of viral particles to sequences in the heparin-binding domain, and interaction of cellular integrins VLA-4 and/or VLA-5 with the RetroNectin molecule.

What are Very Late Antigen-4 (VLA-4) and Very Late Antigen-5 (VLA-5)?
VLA-4 and VLA-5 belong to the integrin superfamily and are also known as integrin α4β1 and integrin α5β1, respectively. Integrins are cell-surface receptors that mediate cell-cell and cell-extracellular matrix interactions.
What cell types can RetroNectin reagent be used with?
RetroNectin reagent is highly effective for cells that express integrin VLA-4 or VLA-5. These integrin molecules can interact with the CS-1 site and the cell-binding domains of Fibronectin. VLA-4-expressing cells include T cells, B cells, monocytes, NK cells, eosinophils, bone marrow monocytic cells, and lymphoid progenitors. Thymocytes, activated T-cells, and mast cells express VLA-5.
What types of viral vectors can be used with RetroNectin reagent?
RetroNectin reagent can be used to enhance retroviral and lentiviral transduction. It is not known if RetroNectin reagent is useful for introducing nucleic acids.
Does RetroNectin reagent work for retroviruses packaged with different envelopes?
RetroNectin reagent can be used to enhance transduction of VSV-g, ecotropic, and amphotropic retroviruses and lentiviruses. In addition, this product is compatible with retroviruses with a GALV-type envelope.
How much can RetroNectin reagent increase transduction efficiency?
When the viral supernatant infection method (mixing of cells and virus, followed by plating with RetroNectin reagent) is used, RetroNectin reagent increases transduction efficiency by 50–70% for human CD34+ cells and by 20–30% for mouse bone marrow mononuclear cells.
How much cytotoxicity is typically observed when using RetroNectin reagent?
Less cytotoxicity has been observed with RetroNectin reagent compared to Polybrene. For example, there is almost no effect on cellular growth when NIH 3T3 cells are grown in medium containing 0.25 mg/ml of RetroNectin reagent.
Can RetroNectin reagent be used in combination with Polybrene?
Do not use both reagents simultaneously. Concomitant use of RetroNectin reagent and Polybrene will decrease gene-transfer efficiency.
What type of tissue culture plate can be coated with RetroNectin reagent?
Non-coated tissue culture plates or dishes should be used. Takara Bio R&D scientists have tested both coated and non-coated plates and found that RetroNectin coating efficiency is reduced by 1/5 for coated plates. Experimental data indicate that transduction efficiency is above 30% when using RetroNectin-coated, non-tissue culture treated plates, but only 1–3% when using RetroNectin-coated tissue culture-treated plates. Six-well BD-Falcon cell-culture plates (Cat. # 351146) are recommended.
Can RetroNectin reagent be coated on plates that are also coated with collagen?
If the use of double-coated plates is necessary, please test the following two coating methods:
- coat with RetroNectin reagent first, then collagen
- coat with collagen first, then RetroNectin reagent
If transfection efficiencies are very low, perform the transduction procedure using a RetroNectin-coated non-tissue culture treated plate as described in the user manual. After infection, incubate for 4 hours in a 37°C, 5% CO2 incubator. Then collect the cells and transfer to the collagen-coated plate and incubate for 2–3 days.
How many times can RetroNectin reagent be frozen and thawed?
RetroNectin solution (1 mg/ml) can be frozen and thawed up to 5 times. Avoid additional freezing and thawing. RetroNectin solution is stable for one year at –20°C or for approximately three weeks at 4°C.
For how long are pre-coated RetroNectin plates stable?
Pre-coated RetroNectin plates can be stored at 4°C and are stable for 1–2 months (although we recommend using them as soon as possible). Do not store pre-coated plates at –20°C.
Does RetroNectin reagent have to be filter sterilized?
RetroNectin reagent is provided as a sterile solution. Therefore, filtration is not necessary.
Does Takara Bio provide a clinical-grade RetroNectin reagent?
Yes. RetroNectin GMP grade (Cat. # T202) is manufactured as a quality-assured product according to guidelines for Good Manufacturing Practice (GMP) for Investigational Products and can be used for ex vivo clinical applications (limited to investigational use only).
Can cell-culture bags (e.g., X-fold bags) be coated with RetroNectin reagent?
Yes, X-fold bags can be coated with RetroNectin reagent. Below is a protocol that has been tested for an X-fold Bag (85 cm2):
- Coat the bag with RetroNectin reagent* overnight at 4°C, or for 2 hours at room temperature.
*Use at least 9 ml of RetroNectin solution (20 µg/ml) per bag. - Wash with PBS (30 ml x 3 times).
- Add Retrovirus (4–6 hours).
- Wash with PBS (30 ml).
- Add cells (1 x 104 cells/cm2).
- Incubate at 37°C for 3 days under 5% CO2.
NOTE: This method may not be applicable for other types/brands of cell culture bags.
Is there a way to improve transduction efficiency?
Below are some tips on how to improve transduction efficiency using RetroNectin reagent.
- We recommend preparing retrovirus supernatant at a high titer (>106 cfu/ml is desirable). To achieve transduction efficiencies of 60–70%, concentrate the retrovirus solution (e.g., measured titer = 1.15 x 106) more than 10-fold and use it for pre-loading.
- Pre-load viral particles onto RetroNectin reagent-coated dishes at 200–250 µl/cm2, and incubate for 4–5 hours at 32°C for best results.
- Ensure that the virus-loaded plate does not dry after discarding the supernatant and washing with PBS. Add target cells in growth medium immediately after removing the supernatant. If the plate dries, the transduction efficiency will decrease remarkably.
After transduction of cells using RetroNectin reagent, what method do you recommend to resuspend the cells?
For cell lines such as HL60 cells and K562 cells, we have verified that cells can be easily collected by pipetting from the RetroNectin reagent-coated dish. However, for several other cell lines, it may be difficult to collect the cells by pipetting without trypsin treatment.
In general, if cells cannot be collected by pipetting, we recommend collecting cells by trypsin treatment 24 hours after transduction and transferring the collected cells into a fresh dish. Then culture the cells in suspension for ~2 days, and collect the suspended cells for flow cytometry.
How should cells be dissociated from RetroNectin-coated plates?
For strongly adherent cells, like fibroblasts, use trypsin-EDTA (without Ca2+ and Mg2+) to dissociate cells.
For weakly adherent (or suspension) cells, use a 0.02% EDTA/PBS solution following the protocol below. Use trypsin to remove these cells only if the EDTA/PBS treatment is unsuccessful; trypsin may damage these cells.
- Following transfection, transfer the supernatant from the plate to a centrifuge tube.
- Wash the plate with PBS to recover non-adherent cells.
- Dissociate adherent cells from the plate using Cell Dissociation Buffer (Thermo Fisher Scientific; enzyme free, PBS-based) following the manufacturer's instructions.
- Combine all obtained cells in one centrifuge tube, and centrifuge to recover the cell fraction.
- Wash cells with HBSS/HEPES twice, collect by centrifugation, and suspend cells in HBSS/HEPES for further use.
After cells are dissociated from a RetroNectin reagent-coated plate using either trypsin or Cell Dissociation Buffer, are the cells likely to re-bind to the same plate? If performing FACS in a 96-well format, should the cells be transferred to a new plate?
Cells dissociated by trypsin or Cell Dissociation Buffer from a RetroNectin reagent-coated plate will likely re-bind to the same plate. We recommend transferring cells to another plate for FACS using a multi-channel pipette.
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.