iDimerize systems journal club

Put a switch on it...
Imagine activating your specific signaling pathway of interest on demand, independently of upstream signals! iDimerize Systems are powerful technologies for creating conditional alleles that can be activated within seconds of adding a small-molecule ligand.
Of the many publications using iDimerize technology, we have selected a few to highlight. Click each study for a summary.
Publications using iDimerize technology
 Dimerization and activation of FGFR in mouse prostate
Dimerization and activation of FGFR in mouse prostate
Research summary
iDimerize inducible homodimerization technology is used to dissect the roles of mammalian fibroblast growth factor receptor (FGFR) sub-class members in causing prostate hyperplasia in mice. The authors show that induced FGFR1 signaling, but not FGFR2 signaling, leads to prostate hyperplasia.
Paper: Freeman, K. W. et al. Inducible prostate intraepithelial neoplasia with reversible hyperplasia in conditional FGFR1-expressing mice. Cancer Res. 63, 8256-63 (2003).

Advantages of inducible dimerization
- Activate one specific FGFR
- Induce at any developmental time point
- Remove ligand to study phenotype reversibility
- Easier setup than cre-lox or regulated transcription systems
Experimental design
Mice with inducible FGFR1 and FGFR2 transgenes under the control of a prostate-specific promoter were created. Each construct encoded a membrane-targeting sequence, the tyrosine kinase domain of FGFR, and two copies of the DmrB domain, which dimerize in the presence of the B/B Homodimerizer ligand.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- After two weeks of regular injections with the ligand, only mice with ectopic FGFR1 signaling developed prostate-specific hyperplasia. Receptor tyrosine phosphorylation was confirmed in both stains, suggesting phenotypic differences were due to signaling events downstream of FGFR activation.
- Authors stopped ligand treatment at various time points to see if continued FGFR1 signaling is required for hyperplasia maintenance. They learned that hyperplasia is reversible prior to extensive neovascularization, providing opportunities for early intervention in prostate cancer.
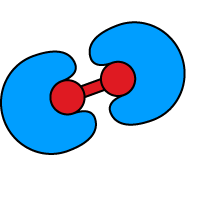
 Recruitment and activation of mammalian Rho GTPase
Recruitment and activation of mammalian Rho GTPase
Research summary
iDimerize inducible heterodimerization technology is used to understand the role of Rho GTPase Cdc42 in regulating actin polymerization. Authors show that induced recruitment of Cdc42 to the membrane is sufficient for producing actin-based membrane protrusions.
Paper: Castellano, F., Montcourrier, P. & Chavrier, P. Membrane recruitment of Rac1 triggers phagocytosis. J. Cell Sci. 2955–61 (2000).
Advantages of inducible dimerization
- Activate one specific GTPase pathway
- Avoid cross-talk between related pathways
- Eliminate the need for upstream stimuli
- Isolate and study transient signaling events
Experimental design
An inducible system for recruiting Cdc42 to the plasma membrane was created in rat basophilic leukemia (RBL-2H3) cells. Two copies of the DmrA domain were anchored to the cell membrane using a signal sequence. The cytosolic, constitutively active form of Cdc42 was expressed fused to a DmrC domain, allowing inducible translocation to the plasma membrane upon addition of A/C Heterodimerizer ligand to growth media.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined experimentally.
Key results
- Localized recruitment of Cdc42 to the membrane was sufficient for producing the signature phenotype of actin-based membrane protrusions.
- A parallel study showed that membrane recruitment of Rac1 triggers actin-dependent particle internalization via phagocytosis (Castellano, F. 2000).
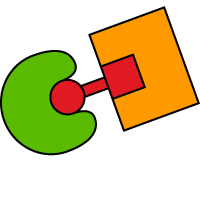
 FasR activation and macrophage apoptosis in mice
FasR activation and macrophage apoptosis in mice
Research summary
iDimerize inducible homodimerization technology is used to rewire the Fas-dependent apoptosis pathway, and generate transgenic mice capable of conditional macrophage ablation. Authors show that induced Fas receptor (FasR) oligomerization leads to systemic macrophage apoptosis.
Paper: Burnett, S. H. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 75, 612-623 (2004).

Advantages of inducible dimerization
- Induction is systemic and reversible
- Induce at any developmental stage
- Remove ligand to study phenotype reversibility
Experimental design
Mafia (Macrophage Fas-induced apoptosis) mice with transgenes under the control of a macrophage-specific promoter were created. The cytoplasmic region of FasR, which mediates DISC assembly, was fused with two copies of the DmrB domain and a membrane-targeting domain. FasR oligomerization was induced by intraperitoneal injections with B/B Homodimerizer ligand.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined experimentally.
Key result
- Mafia mice receiving injections of ligand showed 70–95% loss of macrophages cells following the 5th day of treatment.
 Caspase activation and fat-cell apoptosis in mice
Caspase activation and fat-cell apoptosis in mice
Research summary
iDimerize inducible homodimerization technology is used to rewire the apoptosis pathway and generate transgenic mice capable of conditional ablation of fat cells. Authors show that induced caspase-8 dimerization leads to systemic fat apoptosis.
Paper: Pajvani, U. B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11, 797-803 (2005).

Advantages of inducible dimerization
- Induction is systemic and reversible
- Lacks toxicity of constitutive models
- Induce at any developmental stage
- Remove ligand to study phenotype reversibility
Experimental design
FAT-ATTAC (fat apoptosis through targeted activation of caspase-8) mice with transgenes under the control of adipocytes-specific promoter were created. The catalytic domain of human caspase-8 was fused with a DmrB domain and a membrane-targeting domain. Caspase-8 dimerization was induced by intraperitoneal injections with B/B Homodimerizer ligand.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- FAT-ATTAC mice receiving injections of ligand showed a loss of white and brown fat, plus a marked drop in the adipocyte-specific markers adiponectin and resistin, within 7 days of treatment.
- Mice lost 12% of their total mean body weight by the 10th week of treatment.
- Phenotype recovered to a normal state within 2 months of stopping treatment.
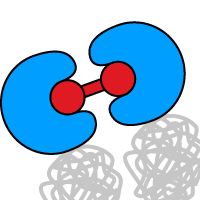 PIP2-mediated ion channel gating at the plasma membrane
PIP2-mediated ion channel gating at the plasma membrane
Research summary
iDimerize inducible heterodimerization technology is used to understand the role of phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis by phospholipase C (PLC), in mediating KCNQ ion channel closure. The authors show that induced PIP2 depletion is sufficient for turning off KCNQ channels, without requiring other PLC-associated signaling molecules.
Paper: Suh, B.-C., Leal, K. & Hille, B. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 67, 224-38 (2010).

Advantages of inducible dimerization
- Allows targeted enzyme recruitment
- Fast induction kinetics (seconds)
- Remove ligand to study phenotype reversibility
Experimental design
Human embryonic kidney tsA-201 cells were created that deplete PIP2 using inositol polyphosphate 5-phosphatase (Inp54p), thus avoiding other signaling molecules generated downstream of PLC. Yeast Inp54p was fused with a DmrA domain and cyan fluorescent protein (CFP). A DmrC domain was anchored at the membrane using a signaling sequence, to allow inducible Inp54p recruitment upon addition of A/C Heterodimerizer ligand to the growth media. A yellow fluorescent protein (YFP)-labeled pleckstrin homology (PH) domain, which naturally binds membrane phosphatidylinositol lipids, was used as a sensor for PIP2 levels.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- In the presence of ligand, Inp54p accumulation at the membrane and a simultaneous release of the fluorescent sensor were observed, confirming PIP2 depletion at the membrane.
- Ligand treatment also caused complete and reversible suppression of KCNQ currents, proving that PIP2 depletion suffices in turning off KCNQ channels.
 Insulin secretion and glucose regulation in diabetic mice
Insulin secretion and glucose regulation in diabetic mice
Research summary
iDimerize inducible reverse dimerization technology is used to create a conditional export system for the controlled secretion of insulin through the endoplasmic reticulum (ER). Authors show rapid and reversible regulation of both insulin and glucose in hyperglycemic mice.
Paper: Rollins, C. T. et al. A ligand-reversible dimerization system for controlling protein-protein interactions. Proc. Natl. Acad. Sci. U. S. A. 97, 7096-101 (2000).

Advantages of inducible dimerization
- Fast (in seconds) and reversible induction
- Plasma-stable ligand inducer
- Uses endogenous export machinery
- Eliminates the need for upstream stimuli
Experimental design
Engineered cells carrying a transgene for inducible insulin secretion were transplanted in hyperglycemic mice. Transgenes expressed human proinsulin fused to four copies of the DmrD domain (two copies are shown in the figure). A furin cleavage sequence was included to allow removal of fusion domains in the trans-Golgi, and release of biologically active protein. In the absence D/D Solubilizer ligand, the DmrD domain self-aggregates, resulting in cargo that is too large for ER export. Ligand treatment solubilizes the aggregates, enabling export.*
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- Cells treated with ligand showed insulin release in a dose-dependent manner.
- Insulin secretion was observed 30 min following treatment and was fully terminated an hour after ceasing treatment.
- Hyperglycemic mice exhibited rapid regulation of both insulin and glucose levels 15 min following ligand administration.
 Induced apoptosis of microglial cells to study retinal degeneration
Induced apoptosis of microglial cells to study retinal degeneration
Research summary
Microglia have been reported to play a central role in chronic degenerative diseases. The iDimerize Inducible Homodimer System was used to specifically deplete bone marrow (BM)-derived microglia cells. This allowed researchers to specifically define the roles of retinal microglia and BM-derived microglia in a mouse model of retinal degeneration.
Paper: Wang, N.-K. et al. Origin of fundus hyperautofluorescent spots and their role in retinal degeneration in a mouse model of Goldmann-Favre syndrome. Dis. Model. Mech. 6, 1113-22 (2013).

Advantages of inducible dimerization
- Induce apoptosis specifically at the appropriate developmental stage
- Induction is systematic
- Easier setup than other inducible systems
- Homodimerizer does not cross the blood-brain barrier; apoptosis is induced in a specific subset of cells
Experimental design
Transgenic rd7 mice (a model for retinal degeneration) were created that contained the transgene for macrophage Fas-induced apoptosis. Apoptosis of microglial cells was activated by dimerization using the B/B Homodimerizer*. Microglia depletion was measured by FACS analysis. Morphological changes and changes in the distribution of microglia was observed using immunohistological analyses. Cytokine expression was determined by RT-qPCR.
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- Loss of BM-derived microglia enhances inflammatory pathways and accelerates retinal degradation. This also leads to decreased retinal rosettes and an enlarged spleen.
- Retinal microglia release inflammatory cytokines-IL-1beta, IL-6, and TNF-alpha. These cytokines increase retinal degeneration.
- B/B Homodimerizer does not penetrate the blood-brain barrier, this leads to specific depletion of BM-derived microglia and preserves retinal microglia.
 The role of the juxtamembrane region in delta EGFR nuclear localization
The role of the juxtamembrane region in delta EGFR nuclear localization
Research summary
How does ΔEGFR/EGFRvIII translocate to the nucleus and regulate gene expression? The iDimerize Inducible Homodimer System was used to investigate the role of the juxtamembrane region in ΔEGFR nuclear localization. The authors show that nuclear translocation of ΔEGFR is critical to its oncogenic potential, independent of its kinase activity.
Paper: Gururaj, A. E. et al. Access to the nucleus and functional association with c-Myc is required for the full oncogenic potential of ΔEGFR/EGFRvIII. J. Biol. Chem. 288, 3428-38 (2013). 
Advantages of inducible dimerization
- Induce at any developmental stage
- Induction is systemic and reversible
- Specifically activate dimerization-dependent kinase activity
Experimental design
Cell lines stably expressing ΔEGFR with a mutation in the juxtamembrane region (chiΔEGFR-NLS1) were created. In these lines, ΔEGFR with an NLS1 mutation (decreasing nuclear localization) was fused to two copies of the DmrB domain. Dimerization was induced using the B/B Homodimerizer* and the kinase activity and localization of chiΔEGFR-NLS1 were observed.
*Studies are presented using iDimerize components. For complete experimental details, please refer to the original publication. The optimal position and copy number of fusion domains varies per study and must be determined empirically.
Key results
- The kinase activity of chiΔEGFR-NLS1 mutants, with decreased nuclear localization, increased upon activation of dimerization. This suggests that kinase activity and nuclear localization of ΔEGFR are independent in glioblastoma cells.
- Loss of nuclear localization of ΔEGFR decreases tumor growth in mice. Even when kinase activity is increased by two weeks of dimerization, ΔEGFR tumorigenic effects are attenuated without localization.
- Using ChIP-seq, c-Myc binding sites (E-box motifs) were found in a greater number of ΔEGFR binding sites. Downregulation of c-Myc/ΔEGFR genes was found to be coupled with decreased tumorigenic potential.
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.