RetroNectin reagent overview
Some cell types, such as hematopoietic cells, are hard to infect even in the presence of high titers of lentivirus or retrovirus. Low transduction efficiency may result from many factors including low viral receptor density, low cell-to-virus contact, the presence of transduction inhibitors, or sensitivity of target cells.
Fortunately, there is a solution—RetroNectin reagent promotes colocalization of lentivirus or retrovirus with target cells to dramatically enhance transduction efficiency.
What is RetroNectin reagent? How does it work?

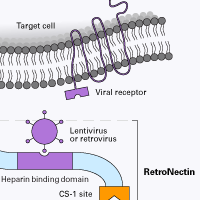
RetroNectin reagent is a recombinant human fibronectin fragment that contains three functional domains: the cell-binding domain (C-domain), the heparin-binding domain (H-domain), and the CS-1 sequence. RetroNectin reagent enhances lentiviral- and retroviral-mediated gene transduction by aiding the colocalization of target cells and viral particles. Specifically, virus particles bind RetroNectin reagent via interaction with the H-domain, and target cells bind mainly through the interaction of cell surface integrin receptors VLA-5 and/or VLA-4 with the fibronectin C-domain and CS-1 sites, respectively. By facilitating close physical proximity, RetroNectin reagent can enhance viral-mediated gene transfer to target cells expressing integrin receptors VLA-4 and/or VLA-5.
Will RetroNectin reagent work for any cell type?
RetroNectin reagent is highly effective for cells that express integrin VLA-4 or VLA-5. VL-4-expressing cells include T cells, B cells, monocytes, NK cells, eosinophils, bone marrow monocytic cells, and lymphoid progenitors. Thymocytes, activated T cells, and mast cell express VLA-5. For transduction, it is important that the target cells express integrin receptors VLA-4 and/or VLA- 5. When using hematopoietic stem cells, cytokine prestimulation may be necessary.
| Cell transduction efficiency with RetroNectin reagent | |
|---|---|
| Cell type | Efficiency of gene transfer (%) |
| TF-1 | 53.3 |
| HEL | 28.1 |
| NIH/3T3 | 80.5 |
| CD34+ BMC* | 86.9 |
| Mouse BM MNC** | 29.2 |
| K-562 | 93.5 |
| HL-60 | 89.4 |
*Bone marrow cells
**Bone marrow-derived mononuclear cells
Does RetroNectin reagent improve transduction when receptor expression is low?
The table below shows the gene expression levels of the GaLV envelope receptor Pit1, integrin VLA-4, and integrin VLA-5 in human hematopoietic stem cells (hCD34+), human adipose-derived stem cells (hADSC), and human mesenchymal stem cells (hMSC). Cells were transduced using various transduction enhancers, including RetroNectin reagent, Polybrene, and Protamine. The expression of virally encoded recombinant fluorescent protein was measured after transduction to assess transduction efficiency. RetroNectin reagent enabled and/or dramatically enhanced viral transduction efficiency, even in stem cells with low expression of GaLV envelope receptor (Pit1), such as hCD34+ and hADSC cells.
| VLA-4, VLA-5, and Pit1 gene expression | |||
|---|---|---|---|
| hCD34+ | hMSC | hADSC | |
| VLA-4 | + | - | + |
| VLA-5 | + | + | + |
| Pit1* | 0.36 | 1.00 | 0.31 |
*Pit1 gene expression relative to hMSC.

Comparison of retrovirus-mediated gene transfer efficiency into human stem cells using various methods.
RetroNectin reagent can be used to enhance transduction of VSV-G, ecotropic, and amphotropic retroviruses and lentiviruses. In addition, this product is compatible with retroviruses with a GALV-type envelope.
How does the use of RetroNectin reagent avoid cell exposure to transduction inhibitors and other toxic substances from viral supernatants?
There are two RetroNectin-mediated infection protocols: the supernatant (SN) infection method and the RetroNectin-bound virus (RBV) infection method. With the SN infection method, cells are mixed with virus supernatant and loaded on a RetroNectin-coated plate. In the RBV method, the retrovirus is first bound to the RetroNectin-coated plate, and cells are added after removing the retrovirus supernatant. In this protocol, removal of the supernatant reduces inhibitory molecules (e.g., molecules secreted from the producer cells such as proteoglycans and/or viral envelope proteins) that can reduce the efficiency of viral-mediated gene transduction.

Schematic protocol for infecting target cells using the RBV method. This protocol can dramatically increase transduction efficiency, especially in hard-to-transduce cells.
Are there published examples of use?
Yes. Click here to view citations.
Do you sell Clinical Grade RetroNectin reagent for ex vivo gene therapy?
Clinical grade RetroNectin reagent for use in ex vivo clinical applications is available for direct purchase by researchers, without the need for a Material Transfer Agreement (MTA). RetroNectin GMP grade (Cat. # T202) is manufactured as a quality-assured product according to guidelines for Good Manufacturing Practice (GMP) for Investigational Products. The Drug Master File for RetroNectin GMP grade has been filed with the U.S. Food and Drug Administration. Clinical grade RetroNectin reagent has been used in over 68 protocols for gene therapy clinical trials at 44 institutions worldwide.
RetroNectin reagent use in gene therapy research.

FAQs about RetroNectin reagent
What is RetroNectin reagent and how does it work? Which cell types can benefit from RetroNectin-assisted viral transduction? Find answers to your questions and more!
FAQs page Learning centerTakara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.