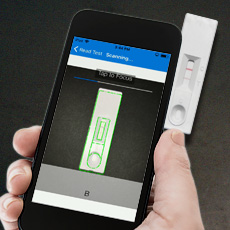
Lenti-X GoStix Plus FAQs
Click on the sections below to find the answers to the most frequently asked questions about our Lenti-X GoStix Plus cassettes and companion app. If you need further assistance, please contact our Technical Support team.
What are the benefits of using Lenti-X GoStix Plus to measure lentiviral titer?
Lenti‑X GoStix Plus cassettes provide a fast and simple method for obtaining an accurate viral titer. Traditional methods for measuring titer such as qPCR, ELISA, or functional assays can be fairly lengthy and cumbersome, and are therefore not ideal for monitoring your supernatant to identify an optimal harvest time, or for testing multiple samples. Lenti‑X GoStix Plus enable you to obtain a titer in 10 minutes by simply adding 20 µl of supernatant to the GoStix cassette and then scanning the cassette with your smartphone camera after a brief incubation. The app also records the titer so that you can email yourself the result for record keeping. The chart below compares the time required to measure lentivirus titer with Lenti‑X GoStix Plus relative to traditional methods.

Figure 1. Most commonly used methods of lentiviral vector titration and their associated timelines (in hours). Lenti‑X GoStix Plus: a lateral flow-based method for the detection of lentiviral p24 in supernatants. qRT-PCR: quantification of viral RNA genomes by qRT-PCR. ELISA: measurement of the amount of p24 capsid protein. PCR: detection of integrated DNA proviruses by qPCR. IFU (FACS): determination of the percentage of infected cells via FACS analysis of RFP/GFP positive cells. IFU (Selection): infectious units determined by quantifying the number of drug resistant colonies in transduced populations.
What is p24?
Recombinant lentiviruses generated for research use contain major structural proteins, including envelope proteins, matrix proteins, and virus core proteins (Figure 2). p24 is a structural protein that comprises a majority of the capsid (or shell) of lentiviral particles, and is encoded by the gag gene (along with the nucleocapsid proteins, p6 and p7, and the matrix protein, p17).
Lenti‑X GoStix Plus and methods such as p24 ELISAs are designed to specifically measure the amount of p24 capsid protein present in lentiviral supernatants, because levels of p24 correlate directly with infectious viral titer.

Figure 2. p24 is a major structural component of the lentivirus capsid.
What is a GoStix Value (GV) titer?
The GoStix Plus app scans the GoStix cassette for the test (T) and control (C) bands, measures the intensity of each band (Figure 3), and then performs a calculation that generates a metric which we refer to as the GoStix Value (GV; equivalent to ng/ml p24). The intensity of the test band on the cassette increases with the amount of p24 protein in your lentiviral supernatant, which in turn correlates with increasing infectious units (IFU). The GV is correlated to infectious titer in the same way that ng p24 or genome copies (gc) correlate to infectious titer when using traditional p24 ELISAs or qRT-PCR assays, respectively, to determine titer (Figures 4–6, see subsequent questions). As is the case with those other assays, the GV that is best for each experimental application will vary depending on several factors including the target cells, the MOI required, and the lentiviral vector and packaging system used.

Figure 3. The GoStix Plus app uses a smartphone camera to determine the relative intensities of the test (T) and control (C) bands and uses the ratio to calculate the titer.
How accurately do GV titers correlate to infectious units (IFU)?
Data presented in Figures 4 and 5 (below) demonstrate a strong correlation between GV and IFU across varying concentrations of sample and varying lentiviral packaging systems.

Figure 4. Lenti‑X GoStix Plus-based titers correlate with transduction titers across varying concentrations. Lenti‑X ZsGreen1 lentivirus was prepared using the Lenti‑X Packaging Single Shots lentiviral packaging system. The sample preparation was serially diluted and added to Lenti‑X GoStix Plus cassettes and densitometric analysis was performed. In addition, each dilution was titrated on HT1080 cells to determine actual IFU/ml. The IFU/ml for the unknown samples was calculated using a GV to IFU/ml ratio determined from a reference virus produced using the same packaging system and the same harvest time as the unknown sample. The IFU/ml calculated from the Lenti‑X GoStix Plus was then plotted against the actual IFU/ml.

Figure 5. Lenti‑X GoStix Plus-based titers correlate with transduction titers across varying packaging systems. ZsGreen1 lentivirus was prepared using three different commercially available lentiviral vector and packaging systems. Samples for all preparations were diluted and added to Lenti‑X GoStix Plus cassettes and analyzed. In addition, each dilution was titrated on HT1080 cells to determine actual IFU/ml. The IFU/ml for each unknown sample was calculated using a GV (ng/ml p24) to IFU/ml ratio determined from a reference virus produced using the same packaging system and the same harvest time as the unknown sample.
Where do I download the app?
You can download the GoStix Plus app from the App Store or Google Play. Search for "GoStix Plus".
Which devices are supported by the GoStix Plus app?
The app works on all Apple iPhones using iOS 13.0 or later, and on Apple iPod Touch using iOS13.0 or later. Android smartphones using Android 9 and up are also supported.
Please note: the GoStix Plus app has not been validated for use with tablets.
How do I correlate GV to IFU/ml or ng p24?
It is as simple as doing a side-by-side comparison. The data presented below compare ELISA- and Lenti-X GoStix Plus-based titers.

Figure 6. Lenti-X GoStix Plus-based titers can be used in a similar fashion to ELISA-based titers. Panel A. Lenti-X ZsGreen1 lentivirus was prepared using the Lenti-X Packaging Single Shots lentiviral packaging system. Percentages of cells transduced (% transduced) were determined on HT1080 cells via FACS analysis. In addition, levels of lentiviral p24 were assessed using the Lenti-X p24 Rapid Titer Kit (ELISA) and Lenti-X GoStix Plus. Transductions were performed on HT1080 cells using a range of MOIs and the resulting transduction efficiencies were plotted against the associated ELISA and GoStix Values (ng/ml p24). Panel B. Fluorescent images of the transduced cells were taken at 48 hours post-transduction.
What factors can impact a GoStix titer?
When using Lenti‑X GoStix Plus (or any other method, e.g., p24 ELISA, or qRT-PCR) to compare titers between different experiments, the following variables must be consistent: lentiviral packaging systems, lentiviral vectors, transfection methods, harvest times, and scanning devices. If any one of these parameters is changed between experiments, we recommend that you re-correlate the GV to IFU.
How can harvest time affect the GoStix Value?
The GoStix Value (GV) is proportional to the amount of p24 protein produced, which in turn correlates with the length of the time to harvest. Therefore, as demonstrated in Figure 7 (below), a longer harvest time will result in a larger GV (ng/ml p24), assuming that other conditions (e.g., packaging system, transfection method, etc.) are kept constant. The data presented below also demonstrate that the ratio between GV and IFU/ml varies depending on the harvest time, which is why harvest time must be kept constant to enable calculation of IFU/ml from GV, and for comparison across experiments.

Figure 7. p24 production and GoStix Value are dependent on harvest time. ZsGreen1 lentivirus preparations were made in duplicate in accordance with the manufacturer's instructions using Lenti‑X Single Shots and harvested at the times indicated. After harvest, samples were applied to Lenti‑X GoStix Plus cassettes and analyzed for GV (ng/ml p24). Virus dilutions were also titrated on HT1080 cells to determine IFU/ml for each time point.
What happens if I do not wait 10 minutes for the signal to develop?
The test will not give consistent results if the full development time is not observed and you will not be able to accurately compare your result to previously tested samples. A warning within the app will appear if the Skip button is pressed before 10 minutes have elapsed on the timer.
Can Lenti-X GoStix Plus be used even in the absence of a smartphone?
Absolutely, however, without scanning the intensity of the band, the result will be qualitative (yes, my prep is good; or no, my prep is not ready) rather than a quantitative titer.

Figure 8. Using Lenti-X GoStix Plus to obtain a qualitative result. The test band is only visible when you have a useable lentiviral titer, so Lenti-X GoStix Plus can be used qualitatively in the absence of a smartphone scan. In the experiment above, a clear band was generated by a dilution containing ~5 x 105 IFU/ml (as measured by flow cytometry of transduced HT-1080 cells).
Why do I NOT need to create a standard curve for Lenti-X GoStix Plus?
For other commonly used titration kits such as p24 ELISAs and pRT-PCR assays, you always need to compare your test sample to a standard curve that you generate. This is not required for Lenti‑X GoStix Plus because the standard curve for each lot of Lenti‑X GoStix Plus is generated at Takara Bio USA, Inc. and loaded onto the app when you open it and connect your mobile device to the internet. In addition to saving you time and money, this also ensures maximum accuracy in GV readings between lots and experiments.
Why do I have to enter the lot number of my kit into the app and where is the lot number found?
In order to generate accurate data, the app requires that you enter the lot number because different lots of Lenti‑X GoStix Plus yield slightly different standard curves (similar to an ELISA). The lot number can be entered by scanning the QR code located on the GoStix cassette pouch, or it can be entered manually (Figure 9).

Figure 9. The kit lot number can be input manually or by scanning the QR code on the packet.
Does my mobile device need to be connected to the internet?
When you first use the app, you will need to connect your device to the internet so that the standard curves for available lots of the kit can be downloaded into the app. The app uses the lot-specific standard curve to calculate the GV. When you purchase a new lot of GoStix, make sure to reconnect your device to the internet so that the standard curves for new lots can applied to your app.
How do I know if my titer is outside the range of the GoStix?
For accurate results, it is important that the intensity of the test band is less than that of the control band. The closer this ratio is to 1, the closer the sample is to exceeding the standard curve. If the ratio is close to 1, perform a 1:2 dilution of your sample and apply the dilution to a fresh cassette, then indicate the dilution factor in the app when scanning, as indicated by the green arrow in the screenshot below.

Is any data saved in the app?
Yes. Your results will be saved in “Result history” view (accessible from the main menu), provided that you do not delete the app. To avoid losing data, we recommend emailing yourself the data by pressing the [Share] button at the bottom of each data set page, as indicated by the green arrow in the first screenshot below. You can also download all of your data to your mobile device by pressing the [Download all] button in the upper-right portion of "Result history", as indicated by the green arrow in the second screenshot below. Instructions for retrieving your downloaded data are provided under a subsequent question, below.

How do I locate result data downloaded to my mobile device?
Please follow the iOS- or Android-specific instructions provided below to retrieve your data after downloading it to your device.
iOS mobile devices using a Mac computer
- Plug iPhone into Mac computer
- Open a Finder window
- In the left-hand navigation, under “Locations”, select your mobile device
- Select files
- Expand the row listing for the GoStix Plus app
- Copy the “Download” folder to your desired destination
- A .csv file containing the downloaded data can be retrieved from the “Download” folder at the specified location
iOS mobile devices using a Windows computer (with iTunes)
- Plug iPhone into Windows computer
- Open iTunes
- Select the phone icon on the iTunes toolbar located in the upper-left corner of the window
- Select "File Sharing" in the left-hand navigation
- From the list, select the GoStix Plus app
- Select the "Download" folder, then click [Save] and specify a destination
- A .csv file containing the downloaded data can be retrieved from the “Download” folder at the specified location
Android mobile devices
- Open the File Explorer app on the mobile device
- Select "Downloads"
- A .csv file containing the downloaded data will be available
Is it possible to use the GoStix Plus app in languages other than English?
Yes. Depending on the language settings for your mobile device, the GoStix Plus app will display text in English, Japanese, Mandarin, or Korean. To override your device settings and have the app display text in English, navigate to the "About" page and select [Reset language settings].
Please note that it may be necessary to select UTF-8 encoding in Excel to properly view characters for languages other than English in the output .csv files containing results data.
GoStix Plus IFU calculator
Calculate the IFU/ml for up to eight unknown samples from a known reference sample and titer values resulting from GoStix Plus tests.
Which are the best web and mobile apps to advance your research? We have your list!
Check out these convenient research tools for PCR, cloning, CRISPR, virus prep, and more.
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.