Traditionally, clinically relevant cell types such as hematopoietic stem cells (HSCs) and T cells have a low transduction efficiency, regardless of the method used to introduce the exogenous DNA. Although there has been demonstrated success in clinical trials using engineered T cells to treat certain types of cancer, favorable outcomes can be limited by the number of cells that acquire and express the exogenous DNA. In one cancer and immune gene therapy technique, adoptive cell therapy (ACT), T cells engineered to express chimeric antigen receptors (CAR) can recognize cancer cells—like those in bone marrow tumors in patients with acute lymphocytic leukemia who are refractory to chemotherapy—and they will eradicate those malignant cells. Although CAR T-cell therapy gives hope to these patients, the typical low transduction efficiency and T-cell expansion rates can compromise the procedure and get in the way of recovery.
To address these bottlenecks, RetroNectin GMP grade reagent improves retroviral- and lentiviral-mediated gene transfer by colocalizing retrovirus particles and target cells. Colocalization is thought to be accomplished by direct binding of viral particles to sequences in the heparin-binding domain and interaction of cellular integrins VLA-4 and/or VLA-5 with the RetroNectin molecule (Hosoi et al. 2014).

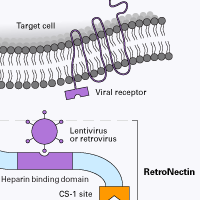
Figure 1. RetroNectin reagent is a recombinant human fibronectin fragment that contains three functional domains: the cell-binding domain (C-domain), the heparin-binding domain (H-domain), and the CS-1 sequence. Specifically, virus particles bind RetroNectin reagent via interaction with the H-domain, and target cells bind mainly through the interaction of cell surface integrin receptors VLA-5 and/or VLA-4 with the fibronectin C-domain and CS-1 sites, respectively. By facilitating close proximity, RetroNectin reagent can enhance viral-mediated gene transfer to target cells expressing integrin receptors VLA-4 and/or VLA-5.
The RetroNectin method is being widely used during ex vivo gene therapy to allow efficient transduction of genes into difficult-to-transduce human cell types. In ex vivo gene therapy, cells are taken from a patient, the disease-fighting gene(s) are introduced, and the modified cells are transplanted back into the patient.

Figure 2. RetroNectin reagent and gene therapy. RetroNectin reagent is also used during expansion culture of T lymphocytes to increase the proportion of naïve T cells. Takara Bio scientists have used RetroNectin reagent for improved T-lymphocyte expansion from peripheral blood mononuclear cells (PBMCs). Using RetroNectin reagent in combination with IL-2 and anti-CD3 antibody improved T-cell expansion in culture (Yu et al. 2008). Moreover, RetroNectin reagent costimulation generated a higher number of T cells with a naïve phenotype than traditional methods. For more data on how RetroNectin reagent can be used to enhance T-cell expansion, read a technical note on this topic.