Whole-genome screens employing CRISPR/Cas9 technology have been recently demonstrated, granting researchers the ability to quickly identify novel gene targets involved in biological pathways of interest (Doench, 2018). CRISPR/Cas9-based screens improve on previous methods, such as RNAi, by providing the ability to completely knock out—rather than knock down—gene function in successfully edited cells, yielding more robust phenotypes that are easier to detect. With the aim of providing a highly effective, user-friendly system for performing genome-wide phenotypic screens in human cell lines, we have applied the latest research regarding sgRNA library design and our expertise in lentiviral systems to develop the Guide-it CRISPR Genome-Wide sgRNA Library System.
Tech Note
Performing a phenotypic screen using the Guide-it CRISPR Genome-Wide sgRNA Library System
Guide-it CRISPR Genome-Wide sgRNA Library System
- Pre-validated sgRNA representation is maintained among transduced cells
- Design principles of the Brunello library are combined with an optimized expression vector and sgRNA-scaffold design to maximize editing efficiency and minimize off-target effects
- Easy-to-use, lyophilized formulation of Cas9 and sgRNA packaging mixes combined with mCherry reporter simplify preparation and titration of viral particles
- 100% editing efficiency confirmed in all randomly selected clones from a Cas9+/sgRNA+ cell population
- Implementation of this system in a functional screen correctly identified genes involved in the purine salvage pathway
Introduction
Library design features
Phenotypic screens using Guide-it CRISPR/Cas9 involve many important processes that determine their effectiveness in identifying novel genes. These include the size of the sgRNA library, the design and selection of individual single guide RNAs (sgRNAs), and optimization of plasmid library amplification, lentiviral packaging, and target-cell transduction processes.
Our library consists of >76,000 sgRNAs targeting ~19,000 protein-coding genes, with four highly active sgRNAs per target (Doench et al. 2016). While it is possible to employ larger libraries, it can be difficult to maintain representation of each sgRNA during amplification as the number of sgRNAs increases. For this reason, some libraries are split into modules, requiring that multiple plasmid amplification, lentiviral packaging, and target-cell transduction steps be performed in parallel, increasing the possibility of bias or loss of sgRNA representation.
The sgRNAs included in our library were chosen from the Brunello library (Doench et al. 2016), which have been shown to have better target specificity with minimal off-target effects to provide an increased probability of identifying relevant genes as compared to other available libraries.
sgRNA representation is one important metric that determines library performance during a screen. An ideal library should have every single sgRNA represented at the exact same quantity, but this is impossible in practice due to variations in sgRNA synthesis and plasmid library amplification. The Guide-it CRISPR Genome-Wide sgRNA Library was synthesized and amplified using our decades of experience working with cDNA libraries to optimize the process, resulting in a very tight distribution of sgRNAs. Each lot is analyzed to confirm that >90% of all sgRNAs in the library are within a 10-fold distribution range (Figure 1, Panel A; sign up to download complete sgRNA representation data).
By providing the library in an easy-to-use format that simplifies lentiviral production and target-cell transduction, our system helps the user maintain sgRNA representation in the transduced cell population without having to deal with the tedious library amplification and virus optimization steps (Figure 1, Panel B).

Figure 1. Representation of sgRNAs within the Guide-it CRISPR Genome-Wide sgRNA Library System. Panel A. sgRNA representation in the starting plasmid population. The Brunello-based sgRNA library was cloned into the pLVXS-sgRNA-mCherry-hyg Vector and amplified. Representation of the sgRNAs within the plasmid DNA was verified by next-generation sequencing (NGS). Bars represent the number of sgRNAs detected at a given read count within the population. Panel B. The correlation between read counts of each integrated sgRNA for the transduced cell population relative to read counts of the corresponding sgRNA for the starting plasmid population. These data show strong Spearman and Pearson correlations, indicating that the Guide-it CRISPR Genome-Wide sgRNA Library System allows for sgRNA representation to be maintained throughout transduction and selection of the target cell population.
Optimized lentiviral sgRNA library vector and sgRNA scaffold design for high editing efficiency
The design for our sgRNA library vector, pLVXS-sgRNA-mCherry-hyg, has been optimized for performance and safety (Figure 2, Panel A). The self-inactivating lentiviral backbones used to deliver Cas9 and the sgRNAs provide puromycin and hygromycin selection markers, respectively, for isolation of transduced cell populations. In addition, the sgRNA library vector also expresses an mCherry reporter for rapid titer determination and visual confirmation of transduced cells. The inclusion of mCherry in the sgRNA vector also makes it easy to determine the optimal multiplicity-of-infection (MOI) required for transduction of the Cas9+ cell line with the sgRNA library (Figure 3). This is important, because ideally each Cas9+ cell should be transduced with only one sgRNA.
The sgRNA scaffold encoded by pLVXS-sgRNA-mCherry-hyg uses an optimized sequence for improved editing efficiency (Figure 2, Panel B). This modified scaffold maximizes the binding affinity between the sgRNA and the Cas9 endonuclease (Chen et al. 2013). Both the Cas9 and the sgRNA vectors are provided premixed with our Lenti-X Packaging Single Shots and Xfect Transfection Reagent. This mixture is lyophilized to produce a formulation that, with only the addition of water, is immediately ready for transfection of the included Lenti-X 293T producer cell line.

Figure 2. Vector and sgRNA scaffold design used in the Guide-it CRISPR sgRNA library. Panel A. pLVXS-EF1a-Cas9-PGK-Puro and pLVXS-sgRNA-mCherry-hyg vector maps showing the lentiviral vector backbone. The vectors are self-inactivating for increased safety during production and use. The pLVXS-sgRNA-mCherry-hyg vector contains both mCherry and hygromycin markers expressed from an IRES-linked bicistronic expression cassette. The sgRNAs, derived from the Brunello library, are expressed from the human U6 promoter and use an optimized scaffold sequence for better Cas9 loading and editing efficiency (Panel B).

Figure 3. Determination of transduction efficiency of the sgRNA library lentivirus using mCherry fluorescence. sgRNA Library-containing lentivirus was produced following the instructions in the user manual: water was added to a tube of sgRNA Library Transfection Mix, vortexed, incubated for 10 min and then added to the provided Lenti-X 293T cells. Lentivirus was harvested after 48 hours and used to transduce Cas9-expressing A375 cells at varying MOIs. Transduced cells were plated and analyzed for transduction efficiency by fluorescence microscopy after 48 hours.
Results
Editing activity of the Guide-it CRISPR Genome-Wide sgRNA Library in randomly selected clones
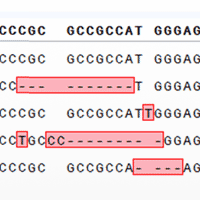
The editing activity of our sgRNA library system was tested by analyzing twenty randomly selected clones from a melanoma cell line (A375) transduced with both Cas9 and the sgRNA library. The sgRNA sequence was amplified from each clone, and the amplicon was sequenced to identify the sgRNA and design a primer set to amplify the sequence of the sgRNA's genomic target. The workflow schematic is shown in Figure 4, Panel A. Editing activity was then assessed using the Guide-it Mutation Detection Kit. The results of this resolvase-based assay are shown in Figure 4, Panel B. Cleavage products were observed for all isolated clones indicating a successful editing event. Each sgRNA found in the clones was also mapped to a correlation plot to determine the relative read counts of the cloned sgRNA within the original plasmid sgRNA pool and transduced cell population (Figure 4, Panel C). The even distribution of the randomly selected sgRNAs within the population suggests that there is minimal bias based on the starting representation level of the sgRNA. To further assess the editing activity in these clones, the amplified target sequences were cloned using the Guide-it Indel Identification Kit for sequencing of the indels. The sequencing results for two representative clones from the tested population, shown in Figure 5, demonstrate typical patterns of indels produced during these types of knockout experiments.

Figure 4. Determination of editing activity of the Guide-it CRISPR Genome-Wide sgRNA Library in randomly selected clones. Panel A. After transduction of Cas9+ A375 cells with the sgRNA library and hygromycin selection, twenty clones were selected randomly and expanded for analysis. Panel B. Genomic DNA was isolated from each clone and analyzed for activity using the Guide-it Mutation Detection Kit. The results of the resolvase assay for 8 representative clones are shown; NTC: non-transduced cells, (+) PCR products treated with Guide-it Resolvase, (–) untreated PCR products. Panel C. The sgRNA sequences from the twenty clones were mapped against the correlation plot of the plasmid library and transduced gDNA. The sgRNAs isolated from the randomly-selected clones are marked with orange dots within the correlation plot of all sgRNAs (blue dots).

Figure 5. Indel identification in edited A375 clones Sequencing data from Cas9+/sgRNA+ A375 cells are shown for two representative clones from the edited population, demonstrating typical indel patterns.
Using the sgRNA library to screen for 6-thioguanine resistance
The efficacy of our lentiviral sgRNA library system was tested by performing a screen for genes involved in the purine salvage pathway. The selective agent, 6-thioguanine (6-TG; a purine analog), is metabolized intracellularly into its active metabolite by the hypoxanthine-guanine phosphoribosyl transferase (HPRT) gene. These metabolites are incorporated into DNA resulting in activation of the DNA mismatch repair system, which subsequently leads to apoptosis. Therefore, cells in which HPRT is knocked out should be able to survive in the presence of 6-TG.
Cas9+ cells were transduced with the sgRNA library virus at a low MOI and selected on hygromycin to select for Cas9+/sgRNA+ cells. The surviving cells were expanded and split into two populations. One was grown in the presence of 6-TG, whereas the other was maintained without selection as a reference-control population. After selection in 6-TG was complete, cells from both populations were expanded, and genomic DNA from each population was purified and analyzed by NGS (Figure 6).

Figure 6. Workflow schematic for a screen using 6-thioguanine (6-TG) selection. After transduction of Cas9+ A375 cells with the sgRNA library, hygromycin selection, and expansion, the Cas9+/ sgRNA+ cells were split into two populations. One Cas9+/sgRNA+ cell population was exposed to 6-TG, while the control population was expanded under normal culture conditions without exposure to 6-TG. After expansion of both cell populations, genomic DNA was harvested and prepared for sequencing with the Guide-it CRISPR Genome-Wide sgRNA Library NGS Analysis Kit.
NGS analysis of the genomic DNA isolated from the cell population selected on 6-TG indicated a substantial loss of sgRNA representation in this population relative to the control population (or relative original plasmid sgRNA library), and a corresponding overrepresentation of all four sgRNAs targeting the HPRT gene (enriched as much as 30,000-fold; Figure 7, Panel B inset). In addition, representation of these guides within the control population was maintained when compared to their representation in the original plasmid sgRNA library (Figure 7, Panel A). Similarly, four sgRNAs targeting another gene, NUDT5 were also found to be over-represented in the screen and were identified as playing a role in the purine synthesis pathway by indirectly providing an eventual substrate on which the protein encoded by HPRT can act (Figure 7, Panel A). These results are similar to those reported by the developers of the Brunello library (Doench et al. 2016).

Figure 7. Identification and analysis of sgRNAs isolated from cells after a 6-TG screen. Panel A. sgRNA representation was compared between the original plasmid library population used to prepare lentivirus, and the resulting transduced cell populations (selected and non-selected). All four sgRNAs targeting HPRT (red dots) and NUDT5 (black dots), respectively, were enriched in the 6-TG-selected population (blue dots). Representation of these sgRNAs in the plasmid library (gray) and transduced, unselected population (orange) is also shown. Panel B. After selection, sgRNA representation (blue) was shifted in response to 6-TG. Individual sgRNAs that were enriched are highlighted within this correlation (red and orange dots). The inset shows fold-enrichment of the four sgRNAs targeting the HPRT gene as compared to the plasmid library.
Conclusions
The system contains lyophilized lentiviral transfection mixes for high-titer production of both Cas9 and sgRNA lentivirus, making it easy to establish and perform highly effective knockout screens while maintaining guide representation. sgRNA representation in every lot of the Guide-it CRISPR Genome-Wide sgRNA Library System is optimized such that >90% of all the sgRNAs in the plasmid library are within a 10-fold distribution range, and this representation is well maintained when the library is transduced into cells.
The optimized vector and sgRNA scaffold design, as well as the highly active sgRNAs, ensure high editing activity in transduced cell populations. In our hands, no bias for higher transduction efficiency was observed for sgRNAs with higher representation within the plasmid library (and vice versa). The pooled-library screen with 6-TG performed using the Guide-it CRISPR Genome-Wide sgRNA Library System and the Guide-it CRISPR Genome-Wide sgRNA Library NGS Analysis Kit successfully identified known key components of the purine salvage pathway.
Methods
sgRNA representation within the library and the transduced cell population
The Brunello-derived sgRNA library was cloned into the pLVXS-sgRNA-mCherry-hyg Vector and amplified. The plasmid DNA was harvested and sequenced on an Illumina® MiSeq® to determine sgRNA representation. The sgRNA-containing lentivirus was produced following the user manual. Briefly, water was added to the Guide-it Genome-Wide sgRNA Library Transfection Mix, vortexed, and added to Lenti-X 293T cells after a 10-min incubation. Lentivirus was harvested after 48 hrs and used to transduce Cas9-expressing A375 cells. Transduced cells were selected on hygromycin (500 µg/ml) for 9 days, at which time genomic DNA was harvested and processed for NGS library preparation. The library was then sequenced on an Illumina MiSeq instrument to determine the relative read counts of each integrated sgRNA relative to the starting plasmid DNA population.
Determination of optimal MOI for transduction with sgRNA library virus
The sgRNA Library-containing lentivirus was produced following the included protocol. Water was added to the Guide-it Genome-Wide sgRNA Library Transfection Mix, vortexed, and added to Lenti-X 293T cells after a 10-min incubation. Lentivirus was harvested after 48 hrs and used to transduce Cas9-expressing A375 cells at varying MOIs to achieve approximately 30–40% transduction efficiency. Transduced cells were plated and analyzed for transduction efficiency by fluorescence microscopy and FACS after 48 hrs.
Resolvase assay for determining editing efficiency in the library population
Genomic DNA was isolated from twenty randomly selected Cas9+/sgRNA+ clones, target sequences were amplified, and PCR products were analyzed for mismatches using the Guide-it Mutation Detection Kit following the kit's protocol.
6-TG assay
A375 cells were transduced to produce a Cas9+/sgRNA+ cell population following instructions provided in the Guide-it CRISPR Genome-Wide sgRNA Library System User Manual. The cells were expanded and split into two populations. One line was selected using 12 µg/ml of 6-TG for 14 days, while the other line was maintained without adding 6-TG. The surviving cells were then expanded, and genomic DNA from the cells was isolated and processed for NGS analysis. The sequencing data were analyzed to determine which guide RNAs had increased or decreased in frequency in response to 6-TG.
NGS analysis
After conducting an sgRNA library screen, cell populations were harvested, genomic DNA (gDNA) was isolated, and sgRNA sequences were amplified from the integrated proviruses contained in the genomic DNA using the Guide-it CRISPR Genome-Wide sgRNA Library NGS Analysis Kit. The amplicons were then sequenced on an Illumina MiSeq platform. After sequencing, reads were trimmed using cutadapt software and the sgRNA sequence (20 bp) was mapped to the reference library or controls and the fold-change in sgRNA frequency determined using CLC Genomics Workbench. Results were then analyzed with Excel to determine which sgRNAs have increased or decreased in frequency due to the selective pressure applied in the screen.
References
Chen, B. et al. Dynamic Imaging of Genomic Loci in Living Human Cells by an Optimized CRISPR/Cas System. Cell 155, 1479–1491 (2013).
Doench, J.G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Doench, J.G. Am I ready for CRISPR? A user's guide to genetic screens. Nat. Rev. Genet. 19, 67–80 (2018).
Related Products
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.