Most hematopoietic cells, including hematopoietic stem cells (HSCs) and terminally differentiated cells (e.g., primary T cells and macrophages), are refractory to viral gene delivery. Inefficient transduction of HSCs is a significant obstacle to research into gene therapy strategies for hematopoietic diseases.
The efficiency of lentiviral and retroviral infection of hematopoietic cells is strongly dependent on various factors, including the cell cycle status of the target cells and cell surface receptor expression. To enhance binding and entry of viruses, cofactors are commonly added during transduction of hematopoietic cells. Cationic polymers (e.g., Polybrene), cationic lipids (e.g., Lipofectin or Lipofectamine), and cationic peptides (e.g., protamine) are used as transduction enhancers that neutralize membrane charge and/or promote virus aggregation. However, many of these additives are highly cytotoxic and often negatively impact cell viability and/or proliferation.
Tech Note
Enhanced transduction of hematopoietic cells
RetroNectin reagent
- Enhanced colocalization
RetroNectin reagent brings virus particles and hematopoietic cells close together
- High-efficiency transduction
Virus-cell colocalization results in high transduction efficiency
- Improved results compared to traditional methods
RetroNectin reagent results in better transduction of hematopoietic cells than conventional protocols that use Polybrene
Introduction
Results
Enhanced virus-cell colocalization
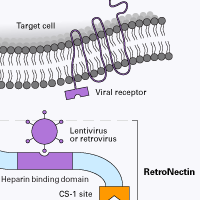
The RetroNectin polypeptide consists of three functional domains derived from human fibronectin protein. RetroNectin reagent enhances transduction by facilitating colocalization of viral particles and hematopoietic cells; virus particles bind to the RetroNectin peptide via interaction with the heparin-binding domain, and hematopoietic cells bind mainly through the interaction of the cell surface integrin receptor VLA-4 with the CS-1 site.
The hypothesized mechanism of RetroNectin-mediated enhancement of transduction is that the cells interact with the RetroNectin molecule via cell surface receptors, and lentiviral and retroviral particles bind to the H-domain of RetroNectin. These interactions increase the localized concentrations of cells and viral particles, an effect that enhances gene transduction.
Learn more about how RetroNectin reagent works »

High-efficiency transduction of hematopoietic cells
By facilitating close physical proximity of cells and viral particles, RetroNectin reagent can enhance viral-mediated gene transfer to a variety of hematopoietic cell types.
| Gene transfer efficiency for hematopoietic cells1 | |
|---|---|
| Cell type | Efficiency (%) |
| Human CD34+ CD38– BMC2 | 95.5 |
| Human PBMC3 | 91.2 |
| TF-1 | 97.9 |
| SupT1 | 97.3 |
| Jurkat | 80.1 |
| K-562 | 90.4 |
| HL-60 | 86.1 |
| Monkey CD34+ BMC | 72.0 |
| Monkey CD4+ T-cell | 85.0 |
1 Transductions were performed using the RetroNectin-Bound Virus (RBV) Method as described in the RetroNectin Recombinant Human Fibronectin Fragment User Manual, which can be found in the product Details, containing Documents, at the bottom of this page.
2 Bone marrow cells.
3 Peripheral blood mononuclear cells.
Improved transduction compared with conventional methods
RetroNectin reagent provides better retroviral and lentiviral transduction efficiency for hard-to-infect cell types (e.g., hematopoietic cells) than conventional transduction protocols, including those that use Polybrene. For human CD34+ cells, RetroNectin reagent can increase transduction efficiency by 50–70% (see example in Figure 1). In addition to improved transduction, virtually no cell toxicity is observed in reactions that use RetroNectin reagent.

Figure 1. Comparison of RetroNectin reagent and Polybrene for retroviral transduction of human HSCs. The efficiency of gene transfer was compared when using either a conventional Polybrene method or RetroNectin reagent for retroviral delivery of GFP to human CD34+ HSCs. Fluorescence was measured by flow cytometry after transduction. GFP expression is plotted on the X-axis of the histograms, and the percentage of CD34+ cells that express GFP is shown on the plot. Transduction efficiency was 86-fold higher when RetroNectin reagent was used than when Polybrene was used (25.8% and 0.3%, respectively).
Conclusions
Research using hematopoietic cells has been limited in part by low efficiency of gene transfer. RetroNectin reagent promotes colocalization of lentivirus or retrovirus with hematopoietic cells to dramatically enhance transduction efficiency.
Methods
The GFP gene was inserted into the BamHI site of the pDON-AI vector (pDON-AI-GFP). This plasmid was transfected into packaging cells to generate recombinant retroviral particles. For transduction, CD34+ HSCs were pre-stimulated with SCF, IL-3, and IL-6. CD34+ cells were infected with the prepared recombinant retrovirus (DON-AI-GFP) either in RetroNectin reagent-coated plates or in the presence of Polybrene. After 48 hours, the percentage of cells expressing GFP was determined using flow cytometry.
Related Products

FAQs about RetroNectin reagent
What is RetroNectin reagent and how does it work? Which cell types can benefit from RetroNectin-assisted viral transduction? Find answers to your questions and more!
FAQs page Learning centerTakara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.