Are you having difficulty with gene transfer into cells that resist transfection and transduction?
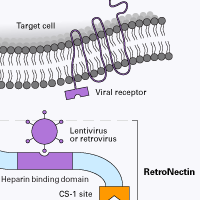
You're not alone. Research using hematopoietic cells and other suspension cells has been limited in part by low efficiency of gene transfer (transduction and transfection) into these cell types. Helping to overcome this obstacle, RetroNectin reagent dramatically enhances transduction efficiency by facilitating close physical proximity of cells and viral particles. This improves viral-mediated gene transfer to target cells expressing integrin receptors VLA-4 and/or VLA-5. VLA-4-expressing cells include T cells, B cells, monocytes, NK cells, eosinophils, bone marrow monocytic cells, and lymphoid progenitors. Thymocytes, activated T-cells, and mast cells express VLA-5.

Don't just take our word for it. Scientists around the globe use RetroNectin reagent for retroviral/lentiviral gene transfer. It holds great promise in clinical applications, such as adoptive cell therapy (T-cell immunotherapy). RetroNectin GMP grade* has been used in over 60 clinical trials.
How much does RetroNectin reagent alone increase transduction efficiency?
When using the viral supernatant infection method (cells and virus are mixed, then plated with RetroNectin reagent), RetroNectin reagent increases transduction efficiency by 50–70% for human CD34+ cells and by 20–30% for mouse bone marrow mononuclear cells.
To support a high viral transduction efficiency, follow these best practices:
- Prepare retrovirus/lentivirus supernatant at a high titer (>106 CFU/ml).
- Pre-load viral particles onto RetroNectin reagent-coated dishes at 200–250 µl/cm2, and incubate for 4–5 hours at 32°C for best results.
- Ensure that the virus-loaded plate does not dry after discarding the supernatant and washing with PBS. Add target cells in growth medium immediately after removing the supernatant. If the plate dries, the transduction efficiency will decrease markedly.
To learn more about RetroNectin reagent and its use in clinical trials, check out our RetroNectin learning center. Happy transduction!
* RetroNectin GMP grade (Cat. # T202) is manufactured as a quality-assured product according to guidelines for Good Manufacturing Practice (GMP).
Back to Blog Front
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.