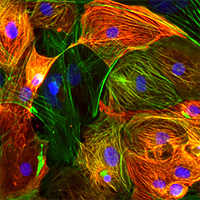
Cellartis hPS cell-derived cardiomyocytes citation list

Takara Bio has over 15 years of experience deriving cardiomyocytes from human pluripotent stem cells under the Cellartis brand. The cardiomyocytes comprise a mixture of atrial, nodal, and ventricular phenotypes, express expected cardiac biomarkers, and exhibit an electrophysiological profile similar to adult primary cardiomyocytes. These features make the cells ideal for drug discovery applications, disease modeling, phenotypic screening, safety pharmacology, and cardiotoxicity testing. Read below for a citation list of studies in which Cellartis stem cell-derived cardiomyocytes were used in peer-reviewed basic, translational, preclinical, and biomedical research.
- da Rocha, A. M. et al. hiPSC-CM monolayer maturation state determines drug responsiveness in high throughput pro-arrhythmia screen. Sci. Rep. 7, 13834 (2017).
The authors cultured Cellartis Cardiomyocytes in conditions that promoted either a fetal-like or mature phenotype. They developed a screening platform consisting of either fetal-like or mature cells and tested various compounds that are known to be low-, intermediate-, or high-risk for causing pro-arrhythmia.
- Higa, A., Hoshi, H. & Takagi, M. Differing responses of human stem cell-derived cardiomyocytes to arrhythmogenic drugs, determined using impedance measurements. Fundam. Toxicol. Sci. 3, 47–53 (2016).
The authors evaluated the arrhythmogenic potential of drugs by testing several types of iPS-derived cardiomyocytes, including Cellartis Cardiomyocytes, using impedance. Cardiomyocytes were cultured and their beating activity was analyzed with the xCELLigence RTCA Cardio System. The cells were treated with arrhythmogenic drugs, and the results showed that the cells displayed drug-induced arrhythmic beating patterns.
- Holmgren, G. et al. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 328, 102–111 (2015).
Cellartis Pure hES-CM (available in Europe and Japan) were used to study doxorubicin-induced cardiotoxicity. The cells' responses were assessed during exposure to doxorubicin for up to 2 days, as well as after a 12-day recovery period. The authors measured the release of lactate dehydrogenase and cardiac-specific troponin T to assess cytotoxicity and cardiotoxicity, as well as performed microarray experiments to identify potential biomarkers for predicting doxorubicin-induced cardiotoxicity.
- Holmgren, G., Sartipy, P., Andersson, C. X., Lindahl, A. & Synnergren, J. Expression profiling of human pluripotent stem cell-derived cardiomyocytes exposed to doxorubicin-integration and visualization of multi-omics data. Toxicol. Sci. 163, 182–195 (2018).
Cellartis Pure hES-CM (available in Europe and Japan) were used to study doxorubicin-induced cardiotoxicity. The cells were exposed to different concentrations of doxorubicin for up to 2 days, followed by a 12-day recovery period. Changes to cell morphology, cardiomyocyte functionality, and proteomic, transcriptomic, and regulatory microRNA networks were observed, and provided insight into mechanisms behind anthracyclin-induced cardiotoxicity.
- Lundqvist, A. et al. The arachidonate 15-lipoxygenase enzyme product 15-HETE is present in heart tissue from patients with ischemic heart disease and enhances clot formation. PLoS One 11, e0161629 (2016).
The authors incubated Cellartis Cardiomyocyctes under normoxic or hypoxic conditions for 6 hours, then used qRT-PCR to determine the effect of hypoxia on ALOX15/15-HETE production in the cells.
- Säljö, K. et al. HLA and histo-blood group antigen expression in human pluripotent stem cells and their derivatives. Sci. Reports 7, 13072 (2017).
During differentiation of the Cellartis iPS cell line ChiPSC22 into Cellartis Cardiomyocytes, HLA and histo-blood antigen expression were measured using flow cytometry and immunohistochemical analysis.
- Sandstedt, M. et al. Hypoxic cardiac fibroblasts from failing human hearts decrease cardiomyocyte beating frequency in an ALOX15 dependent manner. PLoS One 13, e0202693 (2018).
To determine the paracrine effects of fibroblasts on cardiomyocyte beating frequency, human cardiac fibroblasts were cultured under 1% or 21% oxygen for 24 hours. Preconditioned medium from the fibroblasts was mixed with calcium probe mix and added to beating Cellartis Cardiomyocytes. Calcium concentrations and beating frequency were determined for cardiomyocytes cultured in preconditioned media from hypoxic and normoxic fibroblasts.
Learn how to use our cardiomyocytes with the following high-throughput platforms:
FLIPR Tetra system
Perform real-time kinetic assessment of Ca2+ flux and cardiac beating using Cellartis cardiomyocytes with this protocol for the FLIPR Tetra High-Throughput Cellular Screening System.
Patchliner system
Assess electrophysiological behavior using Cellartis cardiomyocytes with this protocol for automated patch clamp recordings on the Patchliner system.
Maestro MEA system
Accurately predict cardiotoxic responses and screen compound efficacy with this protocol using Cellartis cardiomyocytes and Maestro MEA technology.
MED64 MEA system
Record field potentials and deliver stimulation to Cellartis cardiomyocytes using this protocol for the MED64 platform.
CardioExcyte 96 system
Obtain contractility and electrophysiology recordings of Cellartis cardiomyocytes with this protocol on the CardioExcyte 96 system.
xCELLigence RTCA CardioECR system
Collect impedance and EFP recordings from Cellartis cardiomyocytes with the xCELLigence RTCA CardioECR system.
Takara Bio USA, Inc.
United States/Canada: +1.800.662.2566 • Asia Pacific: +1.650.919.7300 • Europe: +33.(0)1.3904.6880 • Japan: +81.(0)77.565.6999
FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES. © 2025 Takara Bio Inc. All Rights Reserved. All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at takarabio.com.